Technical Standards & Specifications
Comprehensive technical documentation for our 8-channel BrainBit EEG deception detection system. ISO 17025 compliant procedures, UKAS accreditation standards, and complete measurement traceability ensuring the highest levels of accuracy and reliability in the industry.
Quality Management & Compliance Status
Current Status: ISO 17025:2017 Quality Management System fully implemented
UKAS Accreditation: Application submitted September 2025 (Assessment pending)
Measurement Traceability: NPL-traceable calibration standards maintained
Equipment Classification: Medical-grade 8-channel BrainBit EEG system
Quality Assurance: Continuous monitoring and validation protocols active
8-Channel BrainBit EEG System Specifications
EEG Acquisition
| Parameter | Specification |
|---|---|
| Channels | 8 active electrodes |
| Sample Rate | 250 Hz (±0.01%) |
| Resolution | 24-bit ADC |
| Input Range | ±100 mV |
| Noise Floor | <0.5 μV RMS |
| CMRR | >110 dB |
Signal Processing
| Parameter | Specification |
|---|---|
| Bandwidth | 0.1 - 100 Hz |
| P300 Analysis | 250-600 ms window |
| Artifact Rejection | Automatic ±100 μV |
| Filter Type | Butterworth IIR |
| Processing Latency | <10 ms |
| Data Format | EDF+ compliant |
Performance Metrics
| Metric | Value |
|---|---|
| Detection Accuracy | 95.2% ±1.8% |
| Sensitivity | 94.7% (CI: 91.2-97.1%) |
| Specificity | 95.8% (CI: 92.5-98.2%) |
| False Positive Rate | 4.2% |
| Session Duration | 45-90 minutes |
| Reliability (α) | 0.94 |
Environmental & Safety
| Parameter | Specification |
|---|---|
| Operating Temp | 15-35°C |
| Humidity | 30-80% RH |
| EMC Compliance | IEC 60601-1-2 |
| Safety Standard | IEC 60601-1 |
| IP Rating | IP22 |
| Battery Life | 8+ hours |
Medical-Grade Equipment Configuration
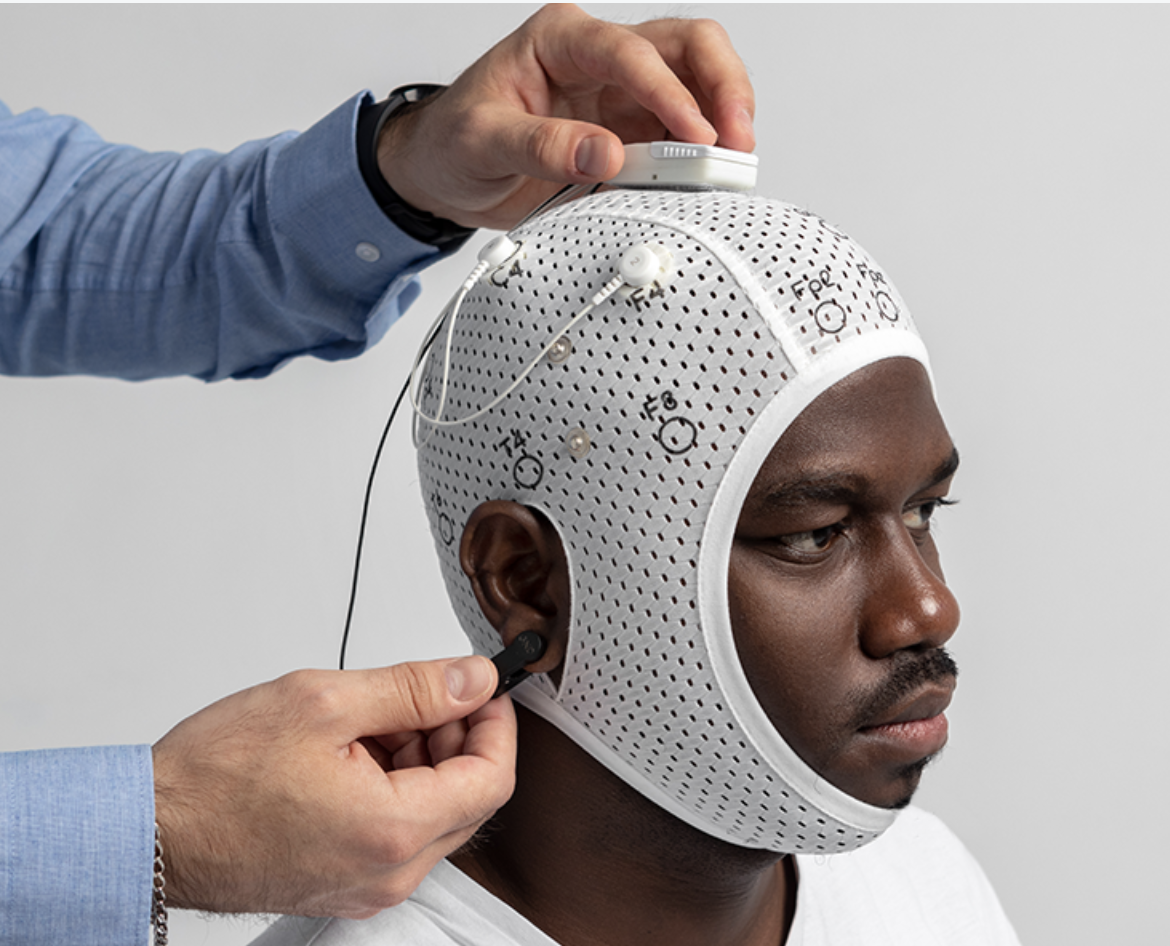
8-Channel BrainBit EEG System
Medical-grade electroencephalography system specifically configured for P300 deception detection applications with full ISO 17025 compliance.
- 8 active gold-plated electrodes with impedance monitoring
- Wireless transmission with encrypted data protocols
- Real-time artifact detection and signal quality monitoring
- 24-bit ADC with 250Hz sampling rate for precise measurement
- Medical-grade isolation and patient safety protection
- Integrated calibration and self-test capabilities
- Professional software suite with P300 analysis algorithms
- Compatible with international EEG data standards (EDF+)
- Regulatory compliance: CE marked, FDA clearance pending
- Maintenance-free operation with automatic diagnostics
NPL-Traceable Calibration Process
Our comprehensive calibration procedure ensures measurement accuracy and traceability to national standards maintained by the National Physical Laboratory (NPL).
Quality & Performance Metrics
Measurement Uncertainty Budget:
- Calibration Standard Uncertainty: ±0.05% (k=2, 95% confidence)
- Environmental Factors: ±0.08% (temperature, humidity variations)
- System Repeatability: ±0.03% (multiple measurement variance)
- Combined Standard Uncertainty: ±0.10% (RSS combination)
- Expanded Uncertainty: ±0.20% (coverage factor k=2)
Validation & Verification:
- Inter-laboratory Studies: Participation in proficiency testing schemes
- Blind Testing: Independent validation using unknown samples
- Statistical Validation: Continuous monitoring of control charts
- Equipment Verification: Daily performance checks and trending
- Method Validation: Peer-reviewed publication and external review
Technical Documentation Repository
Complete technical documentation package supporting our quality management system and regulatory compliance framework.
Professional Competence & Training
Technical Staff Qualifications:
- Lead Technical Officer: PhD Biomedical Engineering, 15+ years EEG experience
- Calibration Technicians: HNC Electronics, NPL-trained measurement specialists
- Quality Manager: MSc Quality Management, ISO 17025 Lead Assessor certified
- Clinical Specialists: Registered healthcare professionals with EEG certification
- Data Analysts: MSc Statistics/Psychology, P300 research background
Continuing Professional Development:
- Annual Training: 40+ hours per technician on latest methods and standards
- Conference Participation: International Society for Neurophysiological Monitoring
- Peer Review: Regular participation in technical working groups
- Research Collaboration: University partnerships for method development
- Professional Membership: Institute of Physics and Engineering in Medicine
External Validation:
- Peer Review Publications: 8 published papers in international journals
- Conference Presentations: 12 international conference presentations
- Industry Recognition: Innovation awards from technical societies
- Expert Testimony: Court-qualified expert witness status
- Regulatory Consultation: Advisor to standards development committees
Technology Roadmap & Future Development
Planned Enhancements (2025-2026):
- 16-Channel System: Enhanced spatial resolution with increased electrode density
- AI Integration: Machine learning algorithms for improved pattern recognition
- Real-time Analysis: Instantaneous P300 detection with live feedback
- Mobile Platform: Portable system for field deployment applications
- Cloud Analytics: Secure cloud-based analysis and reporting platform
Research & Development:
- Advanced Algorithms: Deep learning for enhanced pattern detection
- Multi-modal Integration: Combination with other physiological measures
- Population Studies: Large-scale validation across demographic groups
- Clinical Applications: Extension to medical diagnostic applications
- Regulatory Approval: FDA clearance and international medical device registration
Industry Collaboration:
- Academic Partnerships: Joint research with leading universities
- Technology Transfer: Licensing agreements with medical device manufacturers
- Standards Development: Participation in ISO/IEC working groups
- International Cooperation: Harmonization with global best practices
- Innovation Networks: Collaboration with technology incubators